and Natural Sciences
Renewable energy through photo-electrochemistry
5 October 2023, by MIN-Dekanat
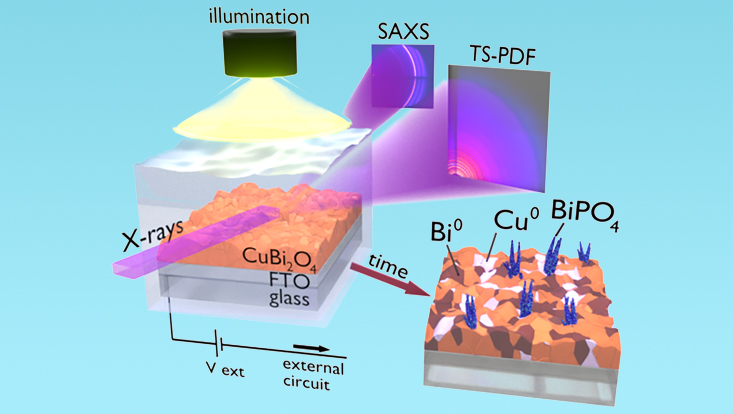
Photo: Davide Derelli
Photo-electrochemistry (PEC) holds the potential to convert renewable energy such as solar light into useful green fuels. However, most known PEC materials suffer from instability issues which are difficult to track and cause a decrease in their performance under continuous operation. A research team from the University of Hamburg (UHH), the Deutsches Elektronen-Sychrotron (DESY) and the Ludwig Maximilian University of Munich (LMU) has developed within a framework of BMBF project LUCENT a new multi-modal setup that determines the structural changes affecting PEC material under realistic operation conditions. The researchers have published their results in the current issue of the journal "Angewandte Chemie International Edition".
One of the most pressing challenges facing modern society is the transition from fossil fuels to green, renewable alternatives. Through photo-electrochemical water splitting, solar energy can be used to convert water directly into its two components, oxygen and hydrogen. Hydrogen holds a great value, as it represents a common building block to the industrial production of many chemical compounds. Moreover, hydrogen can be stored, transported, and burned on demand as a green fuel which produces heat and an utterly harmless waste product – water. However, at present no PEC material succeeded in being transferred from the lab into a real-world working device.
One of the main reasons for the slow rise of efficient photoelectrochemical technology is its low stability in continuous operation." The conditions enabling photo-electrochemical processes to occur are rather harsh," explains Dr Francesco Caddeo from Universität Hamburg. "The use of solar radiation, the application of external voltage and the presence of chemical ions in the electrolyte determine a quick degradation of most photo-electrochemically active materials over time. While many of these degradation phenomena are still largely unknown, revealing them constitutes an essential step toward developing more stable and efficient PEC materials."
“From a chemical perspective, photo-electrochemical water splitting constitutes a rather complex process. To understand the different phenomena, the use of complementary techniques which can “see” the problem from different points of view is crucial,” explains Dorota Koziej, professor at the Department of Physics and member of the Cluster of Excellence "CUI: Advanced Imaging of Matter" at Universität Hamburg. “Some techniques, such as spectroscopy, target specific chemical species which might form at the surface of the material or within the electrolyte. Conversely, X-ray scattering offers an overall perspective of the atomic arrangement in PEC material. The key is exploiting the benefits of each analysis to reconstruct the photo-degradation process as if we were on a crime scene."
However, the time required to perform the analytical measurement also constitutes a major factor to account for. “When we started studying the PEC properties of CuBi2O4 films we immediately realized that a fast photo-degradation process was occurring, which determines a loss of around 90 % of the material performances in just few minutes of operation.” reveals Davide Derelli, from Universität Hamburg and one of the leading scientists of the study. “We therefore turned to the use of the high-brilliant X-ray radiation provided by the X-ray radiation source PETRA III at DESY to collect scattering patterns with high time resolution, which allowed to closely follow the dynamics of the photodegradation process.”
Kilian Frank from LMU explains the experiment: “When the X-rays interact with the material surface, all the radiation is scattered at different angles, creating characteristic patterns. At low angles, the scattering patterns contains information about the outer shape of the PEC film, while at higher angles it reveals its atomic arrangement. To collect both information simultaneously, we used two different detectors, which provided an exceptionally comprehensive representation of the material structure during PEC operation”.
Prof. Koziej already looks to the next scientific challenges: "Understanding the degradation of PEC materials under operation represents only the first step. The objective is to develop new strategies to increase both the stability and the efficiency of PEC devices."
Profile in Angewandte Chemie International Edition

A profile of Prof. Koziej can also be found in the current issue of "Angewandte Chemie International Edition":
Original-Publication
Photodegradation of CuBi2O4 Films Evidenced by Fast Formation of Metallic Bi using Operando Surface-sensitive X-ray Scattering,
D. Derelli*, F. Caddeo*, K. Frank, K. Krötzsch, P. Ewerhardt, M. Krüger, S. Medicus, L. Klemeyer, M. Skiba, C. Ruhmlieb, O. Gutowski, A.-C. Dippel, W. J. Parak, B. Nickel, and D. Koziej,
Angew. Chem. Int. Ed. (2023) *equal first author contributions
https://doi.org/10.1002/anie.202307948